Introduction
The Conference of the Parties to the United Nations Framework Convention on Climate Change (COP) began in 1995. The Kyoto Protocol was adopted at the 3rd COP Conference in 1997; numerous international conferences have been held since then. Amid these actions, the world’s perception of climate change has undergone change.
In December 2015, the Paris Agreement was adopted on the base of technical viewpoints provided by the IPCC. Under this agreement, a long-term goal was set to constrain temperature rise to within 2°C of the pre-industrialization level; initiatives targeting this goal are underway in countries and industries worldwide. In the automobile industry, too, the advance of electrification and the development of hydrogen fuel to achieve carbon neutrality are the subject of attention. The development of synthetic fuels (e-fuels) usable with existing infrastructure and systems is also moving forward. I would like to focus on technical aspects of e-fuels and discuss related issues below.
The e-fuel generation process
E-fuels require a reduction reaction that removes oxygen from carbon dioxide (CO2). As the chemical energy of CO2 is the lowest and most stable among hydrocarbons, it is necessary to provide energy in some form to effect the reduction, and efficiency varies with the generation process used. As an example, a process that ① decomposes water (H2O) to generate hydrogen (H2) and ② uses the H2 as a reducing agent to reduce CO2, to synthesize methane (CH4) from the two stages ①+②, is compared with ③ a process that directly generates CH3 from CO2 and H20. The total energy required for ①+② is the same as that required for ③, but as ② is an exothermic 100% recovery and reuse of the energy generated by the reaction is a necessary condition, ③ is more advantageous in energy utilization efficiency. While this is a simple example, simplification of the generation process and increase in energy efficiency are important issues in the practical application of e-fuels.
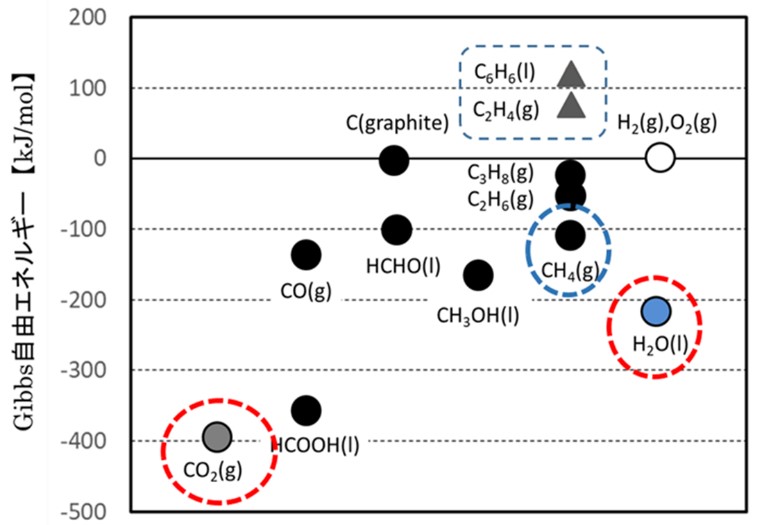
Thermodynamic stability of key substance.
* Gibbs free energy: A metric indicating the thermodynamic stability
of a substance and the direction of a chemical reaction.
Of the substances in the above table, CO2 is the most stable,
requiring a large amount of energy for reduction.
Development of catalysts
Substances including methane and methanol are generated following the reduction of CO2. However, suitable catalysts must be developed in order to advance the chemical reaction so that the intended reaction products are produced after reduction. Copper-based catalysts have traditionally been used for the synthesis of methanol, but the reactions must be performed at high temperature, and carbon monoxide (CO) and CH4 may be unintentionally generated in the reaction process. The development of catalysts that enable reactions under low-temperature and low-pressure conditions has become an issue in efficiently extracting the reaction product following reduction. A promising catalyst using iridium has recently been discovered.
The need for low-cost hydrogen procurement and renewable energy]
Technology for generating methanol and further chemical products from H2 and CO2 has been established to a degree, with hydrogen the likely reducing agent in many cases. Accordingly, the generation and procurement of low-cost hydrogen is a key issue. In Japan, technological development is advancing in areas including the production of H2 through direct decomposition of CH4, with the aim of building a commercial-scale hydrogen supply chain by around 2030, cutting the cost of producing hydrogen without emission of CO2 to 1/10 by around 2050, achieving the cost level of natural gas and other existing forms of energy. Moreover, given the need for CO2 emissions reductions from an LCA standpoint of achieving carbon neutrality, and given the need for large amounts of energy for the above-noted decomposition of CO2, expanding the use of renewable energy is also a key incidental issue.
Summary
Adaptations to climate change and carbon neutrality are being undertaken as a major trend in industry, and involve fields such as finance as well. The adoption of e-fuels by the automobile industry is one such change in response to the trend, and faces many issues in the leadup to practical use and commercialization. Technological issues are particularly vital, and the search for solutions will naturally involve difficulties, but given the significance of carbon neutrality, we hope that the technology will advance in directions that lead to the creation of new industries.
<Reference materials>
- “Report on Survey of Carbon Dioxide Resource Recycling,” Center for Research and Development Strategy, Japan Science and Technology Agency
- “Carbon Recycling Technology Roadmap,” Ministry of Economy, Trade and Industry
- “Breakthrough Environmental Innovation Strategy,” Resolution of the Council for Science, Technology and Innovation
- “Bottlenecks in CO2 Utilization and the Direction of R&D,” Cabinet Office Bottleneck Study Group
- “Development of a catalyst able to synthesize methanol from carbon dioxide at low temperatures,” Article by the National Institute of Advanced Industrial Science and Technology